- Home
- Letter to President Obama
- Acknowledgements
- Executive Summary
- Preface
- Part 1: The Case for HPV Vaccination
- Millions in the U.S. Are Infected with HPV
- HPV-Associated Diseases
- HPV Vaccines
- Part 2: Urgency for Action
- Part 3: Accelerating HPV Vaccine Uptake in the U.S.
- Part 4: Increasing Global HPV Vaccination
- Part 5: High-Priority Research
- Conclusions
- Appendices
- Acronyms
HPV Vaccines
VACCINES PREVENT HPV INFECTIONS AND ASSOCIATED DISEASES
CLINICAL TRIALS DEMONSTRATE HPV VACCINES' POTENTIAL
HPV VACCINES ALREADY ARE BENEFITING MANY
HPV VACCINES ARE SAFE
VACCINES PREVENT HPV INFECTIONS AND ASSOCIATED DISEASES
Two vaccines—Cervarix® and Gardasil®—are approved by the U.S. Food and Drug Administration (FDA) for prevention of several HPV-associated diseases (Table 3). Cervarix® protects against the two types of HPV most commonly found in cancers: HPV16 and HPV18. In addition to protecting against these two cancer-causing HPV types, Gardasil® protects against HPV6 and HPV11. While these two forms of the virus do not cause cancers, they are responsible for most cases of genital warts and RRP. Both vaccines are highly effective at preventing HPV infections and the diseases caused by the HPV types they target. HPV16 and HPV18 cause about 75 percent of cervical cancers in the U.S. and even higher proportions of noncervical cancers associated with HPV, which implies that most HPV-associated cancers potentially could be prevented by these vaccines (Table 2).[1]
How HPV Vaccines Work
Both Gardasil® and Cervarix® are made using recombinant DNA technology. This technology is used to generate viral proteins capable of self-assembling into so-called virus-like particles (VLPs). VLPs are made for each HPV type targeted by the vaccines. HPV VLPs contain no viral genetic material and thus are not infectious but effectively mimic exposure to HPV, provoking the immune system to generate antibodies against specific types of HPV. These antibodies protect vaccinated individuals against infection with target HPV types.
Sources: Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260-70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20833377; Garland SM, Smith JS. Human papillomavirus vaccines: current status and future prospects. Drugs. 2010;70(9):1079-98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20518577
Table 2
U.S. Cancers Attributed to HPV
| Cancer Site | Average # Cancers Per Year at Site (a) | Percent Probably Caused by HPV (a) | Number Probably Caused by HPV (a) | Percent HPV Cancers Probably Caused by HPV16 or 18 (b) | Number Probably Caused by HPV16 or 18 |
|---|---|---|---|---|---|
| Anus | 4,767 | 93 | 4,500 | 93 | 4,200 |
| Cervix | 11,967 | 96 | 11,500 | 76 | 8,700 |
| Oropharynx | 11,726 | 63 | 7,400 | 95 | 7,000 |
| Penis | 1,046 | 36 | 400 | 87 | 300 |
| Vagina | 729 | 64 | 500 | 88 | 400 |
| Vulva | 3,136 | 51 | 1,600 | 86 | 1,400 |
| TOTAL | 33,371 | 25,900 | 22,000 |
(a) Centers for Disease Control and Prevention. Human papillomavirus-associated cancers—United States, 2004-2008. MMWR. 2012 Apr 20;61(15):258-61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22513527
(b) Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036-46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18980286
Table 3
HPV Vaccines
| Gardasil® | Cervarix® | |
|---|---|---|
| HPV Types | 6, 11, 16, 18 | 16, 18 |
| Manufacturer | Merck & Co. | GlaxoSmithKline |
| Initial U.S. Licensing | 2006 | 2009 |
| Approved for Prevention of |
Cervical cancer and precancers Vulvar cancer and precancers Vaginal cancer and precancers Anal cancer and precancers Genital warts |
Cervical cancer and precancers |
| Approved for Use in |
Females (9 to 26 years old) Males (9 to 26 years old) |
Females (9 to 25 years old) |
Sources: U.S. Food and Drug Administration. Gardasil [Internet]. Silver Spring (MD): FDA; 2011 Oct 21 [cited 2013 Aug 1]. Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042; U.S. Food and Drug Administration. Cervarix [Internet]. Silver Spring (MD): FDA; 2013 Aug 1 [cited 2013 Aug 1]. Available from: http://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm186957.htm
CLINICAL TRIALS DEMONSTRATE HPV VACCINES' POTENTIAL
Clinical trials have shown that HPV vaccines could prevent the majority of cervical cancers if used optimally. Of 10,000 young women who were vaccinated as part of clinical trials before they were exposed to cancer-causing forms of HPV, none developed high-grade HPV16/18-associated cervical lesions, which are precursors to invasive cancer.[2,3] (See How HPV Infections Cause Cancers.) Since these lesions are on the pathway to cervical cancer, the striking results led experts to estimate that universal uptake of available HPV vaccines likely would prevent more than two-thirds of cervical cancers worldwide (virtually all of those caused by HPV16/18).[4] Gardasil® prevents other HPV16/18-associated anogenital precancers and HPV6/11-associated genital warts with similar efficacy.[3,5] Neither currently approved HPV vaccine is licensed for prevention of oropharyngeal cancers. However, based on what is known about the biology of this disease, it is highly likely that the vaccines will provide protection against oropharyngeal cancers.[6] Women who received Cervarix® as part of a clinical trial had much lower prevalence of oral HPV infection than participants in the trial who had not received the HPV vaccine.[7]
How HPV Infections Cause Cancers
HPV infection of cervical cells initiates a series of molecular events that sometimes, but not always, results in invasive cancer. Although less research has been done on other HPV-associated cancers, the progression from infection to cancer appears to occur through analagous processes. Many HPV infections are cleared by the immune system. Some of these transient infections result in low-grade precancerous lesions consisting of a few abnormally sized and oddly shaped cells. Many of these lesions disappear within a few months with no treatment. In some cases, persistent HPV infection causes high-grade precancerous lesions. These precancerous lesions sometimes regress spontaneously or can be treated if detected. However, some will progress to invasive cancer. The interval between cervical HPV infection and invasive cancer is usually at least 20 years; however, precancerous lesions usually develop much earlier. Detection and treatment of precancerous lesions through screening has reduced cervical cancer incidence and mortality in the United States and other high-income countries over the past several decades. HPV vaccines have potential to further reduce the burden of this disease by preventing both precancerous lesions and invasive cancers.
Sources: Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11-22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17186016; Crow JM. HPV: the global burden. Nature Outlook. 2012;488(7413):S2-3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22932437
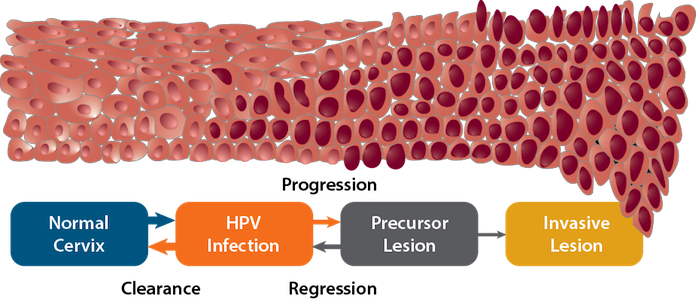
Modified from: Crow JM. HPV: the global burden. Nature Outlook. 2012;488(7413):S2-3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22932437
HPV VACCINES ALREADY ARE BENEFITING MANY
While clinical trial results are important, the value of the HPV vaccines depends on their effectiveness in preventing diseases in real-world settings, as is true of the impact of hepatitis B vaccines on liver cancer rates. It may take decades for the full impact of HPV vaccination on cancer rates to emerge, given the long interval between initial infection and development of invasive cancer (see How HPV Infections Cause Cancers). However, earlier endpoints, including HPV infection prevalence and genital warts incidence (for Gardasil®), can provide critical insight into effectiveness of the vaccines. Measuring these endpoints poses challenges. Monitoring HPV infection requires sampling from the site of infection, DNA extraction, and identification of specific HPV types. In addition, genital warts cases are not consistently reported in many countries. However, investments in these types of surveillance activities are critically important.
Data collected through surveillance have revealed dramatic declines in HPV infection rates and genital warts among vaccinated age groups in the United States, and other countries with HPV vaccine programs provide early indicators of vaccine efficacy.[8-11] In the United States, rates of cervical infection with HPV types covered by the vaccines fell by more than 50 percent among girls 14 to 19 years of age in the four years following vaccine introduction (Figure 3). This study included both vaccinated and unvaccinated girls.[8] The decline was even more dramatic in Australia, where the national HPV program has achieved higher levels of vaccination than in the United States (see Part 4). In that country, the prevalence of infections with HPV types covered by the vaccines fell by more than 75 percent among women 18 to 24 years of age.[9] The prevalence of genital warts in Australia also plummeted; a 92 percent reduction was observed among females under 21 years of age.[10]
Figure 3
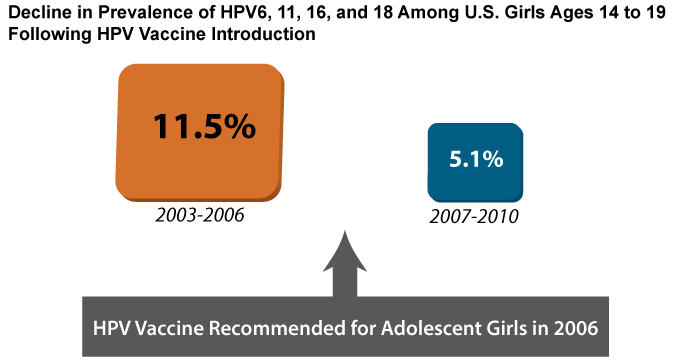
Source: Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208(3):385-93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23785124
"Herd immunity" is the reduced incidence of infections among unvaccinated individuals. High rates of vaccination make it less likely that infections will be spread. Another term used to describe this is "community immunity."
HPV VACCINES ARE SAFE
By early 2013, more than 56 million HPV vaccine doses had been administered in the United States.[13] HPV vaccines have excellent safety profiles, similar to those of other licensed adolescent vaccines. Safety data on the vaccines are drawn from both prelicensure clinical trial data and postlicensure safety monitoring conducted by CDC, FDA, and vaccine manufacturers. While there is always the possibility for an individual to have a serious reaction to an HPV vaccine, as is the case with any medical procedure, the risk of such a reaction is extremely small. To date, three population-based safety studies have been conducted in the United States.[14-16] These studies have identified no serious safety concerns, although one observed increased risk of syncope (fainting) on the day of vaccination and skin infections in the two weeks following vaccination.[16]* The risk of syncope is not unique to HPV vaccination; immunizations in general have been linked to syncope, particularly among adolescents.[17,18] FDA and CDC continue to monitor the safety of the HPV vaccines and follow up on individual reports of serious adverse events. Additional information on safety monitoring of these vaccines can be found on the CDC website.[19]
Footnotes
* Skin infections are not infections with HPV (HPV vaccines do not contain live virus). Medical record review suggested that some of the reported skin infection cases may have been local injection site reactions. Other documented infections (not necessarily at injection site) included impetigo, pilonidal cyst, and carbuncle
References
- Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036-46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18980286
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89-99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22075171
- Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20139221
- Schiffman M, Wacholder S. Success of HPV vaccination is now a matter of coverage. Lancet Oncol. 2012;13(1):10-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22075169
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576-85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22029979
- Gillison ML, Alemany L, Snijders PJ, Chaturvedi A, Steinberg BM, Schwartz S, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(5 Suppl):F34-54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23199965
- Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23873171
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208(3):385-93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23785124
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206(11):1645-51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23087430
- Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23599298
- Hariri S, Markowitz LE, Dunne EF, Unger ER. Population impact of HPV vaccines: summary of early evidence. J Adolesc Health. 2013;53(6):679-82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24263069
- Kahn JA, Brown DR, Ding L, Widdice LE, Shew ML, Glynn S, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130(2):e249-56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22778297
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR. 2013;62(29):591-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23884346
- Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29(46):8279-84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21907257
- Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271(2):193-203. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21973261
- Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012;166(12):1140-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23027469
- Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Pediatr Adolesc Med. 1997;151(3):255-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9080932
- Centers for Disease Control and Prevention. Frequently asked questions on syncope after vaccination [Internet]. Atlanta (GA): CDC; [updated 2011 Feb 8; cited 2013 Aug 15]. Available from: http://www.cdc.gov/vaccinesafety/Concerns/syncope_faqs.html
- Centers for Disease Control and Prevention. Human papillomavirus (HPV) vaccine [Internet]. Atlanta (GA): CDC; [updated 2013 Jan 24; cited 2013 Feb 3]. Available from: http://www.cdc.gov/vaccinesafety/vaccines/HPV/Index.html


